Calcium Bohr diagram Calcium
What is Bohr's Model of an Atom? The Bohr model of the atom was proposed by Neil Bohr in 1915. It came into existence with the modification of Rutherford's model of an atom. Rutherford's model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons.

Calcium atom bohr model Royalty Free Vector Image
Immediately before 1913, the Rutherford model conceived of an atom as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii. Britannica Quiz Matter and More Quiz How does Niels Bohr's atomic model work?
Calcium Atom on a White Background Stock Illustration Illustration of neutron, science 51974420
The Bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Bohr described the hydrogen atom in terms of an electron moving in a circular orbit about a nucleus.. Suggest a reason for the observation that the spectrum of calcium is more complicated than the spectrum of.
Plakat Bohr model representation of the calcium atom, number 20 and symbol Ca. Conceptual vector
Calcium (Ca) atom electron configuration (Bohr model) Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund's principle, and Pauli's exclusion principle. Calcium atom electron configuration through orbit Scientist Niels Bohr was the first to give an idea of the atom's orbit.
Calcium (Ca) AMERICAN ELEMENTS
In this video we'll look at the atomic structure and Bohr model for the Calcium atom (Ca). We'll use a Bohr diagram to visually represent where the electrons are around the nucleus.more.

Electron Configuration for Calcium (Ca, Ca2+ ion)
One of the weaknesses of Bohr's model was that he could not offer a reason why only certain energy levels or orbits were allowed. Figure 10.4.1 10.4. 1: The energy levels of the electrons can be viewed as rungs on a ladder. Note that the spacing between rungs gets smaller at higher energies (CC BY-NC; Ümit Kaya)

modelo bohr del átomo de calcio. vector de stock (libre de regalías) 1951262071 Shutterstock
The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to atomic theory.
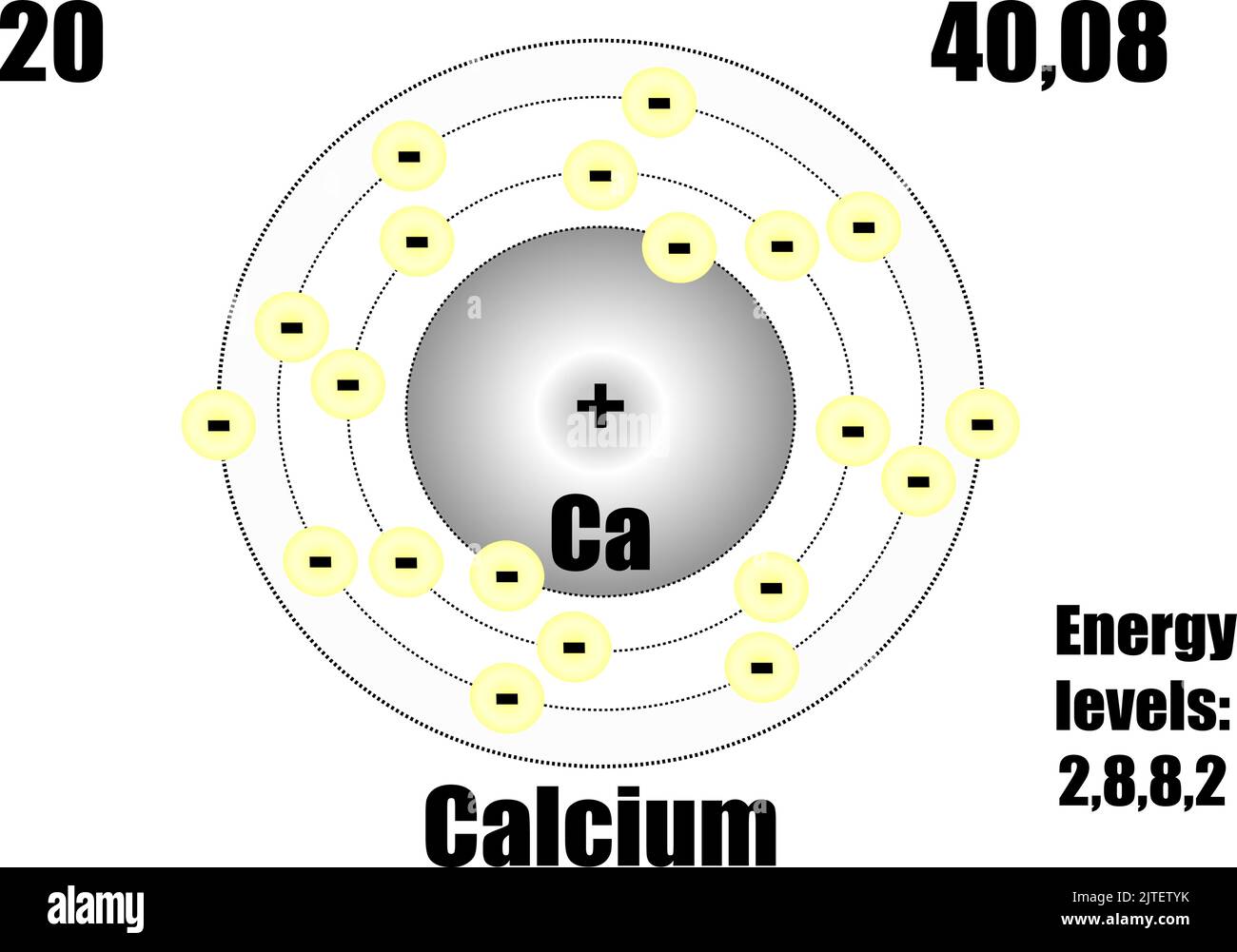
Calcium atom, with mass and energy levels. Vector illustration Stock Vector Image & Art Alamy
Learn how to draw Bohr Models of atoms to further your fundamental understanding of Chemistry.

Calcium Facts
Since Bohr's model involved only a single electron, it could also be applied to the single electron ions He +, Li 2+, Be 3+, and so forth, which differ from hydrogen only in their nuclear charges, and so one-electron atoms and ions are collectively referred to as hydrogen-like atoms.The energy expression for hydrogen-like atoms is a generalization of the hydrogen atom energy, in which Z is.
Bohr Model Atom Electron Configuration Argon Calcium Molecular Transparent PNG
An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or 'shells', around the central nucleus. This is sometimes called the Bohr, or the 'solar system', model. Download this
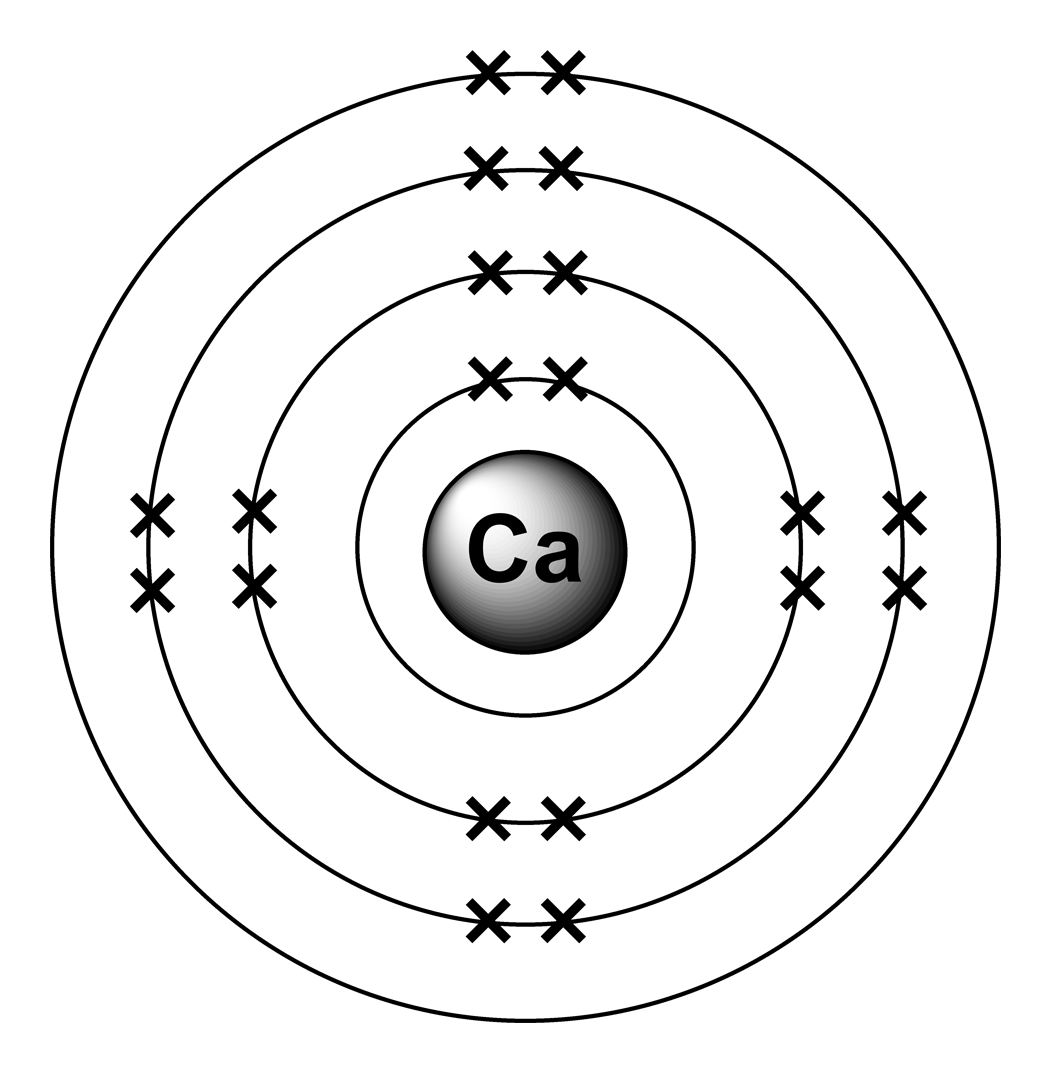
Bohr Diagram Of Calcium
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.

Calcium Atom Model 3d
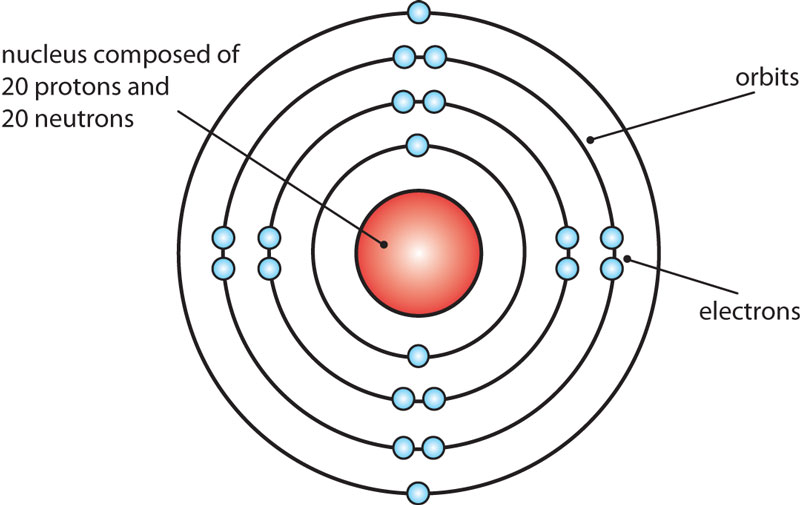
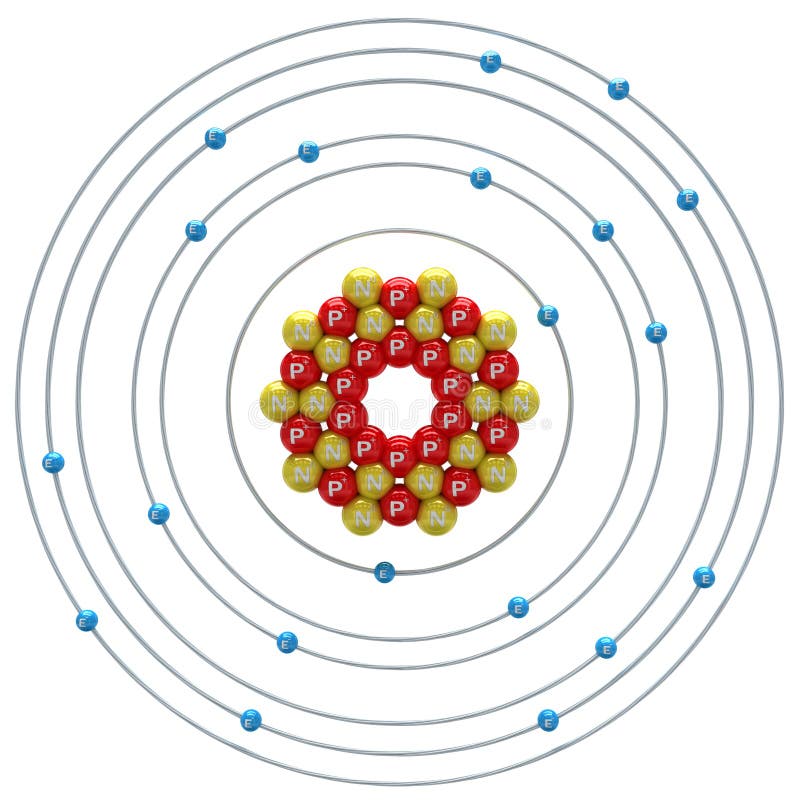
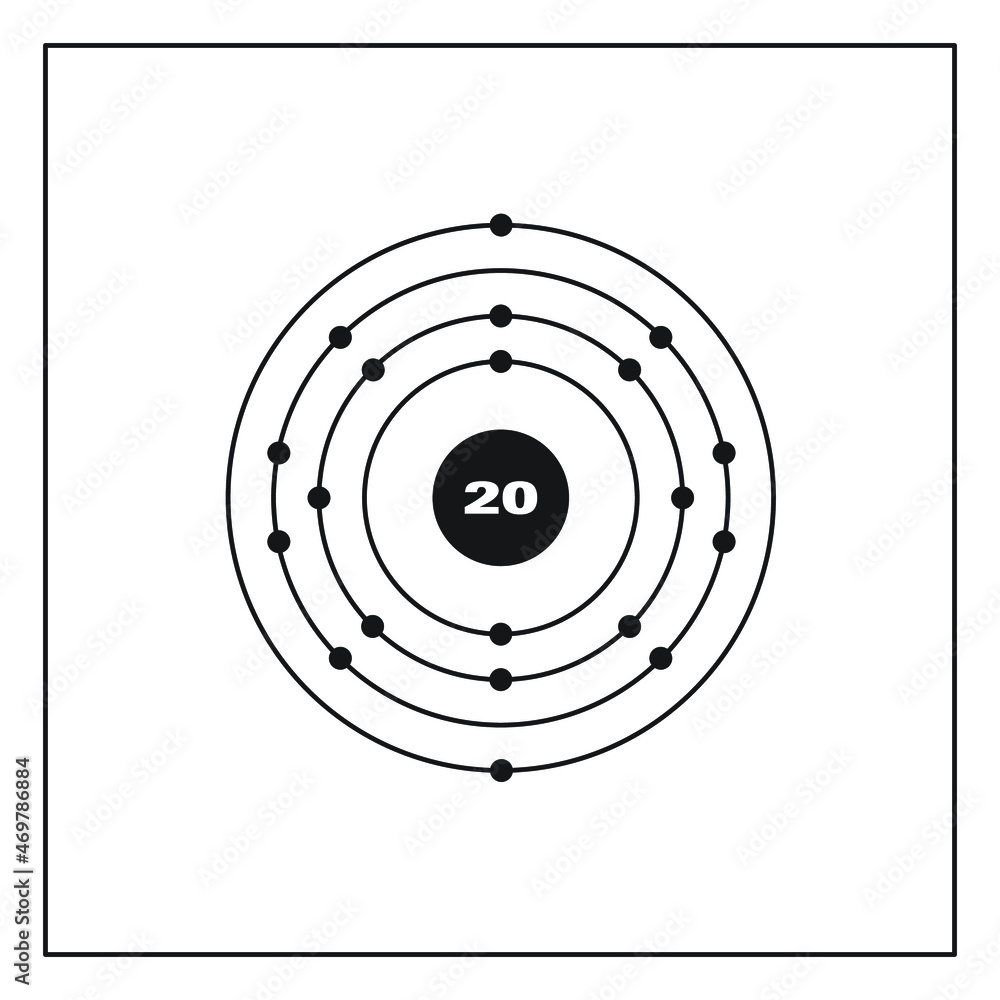
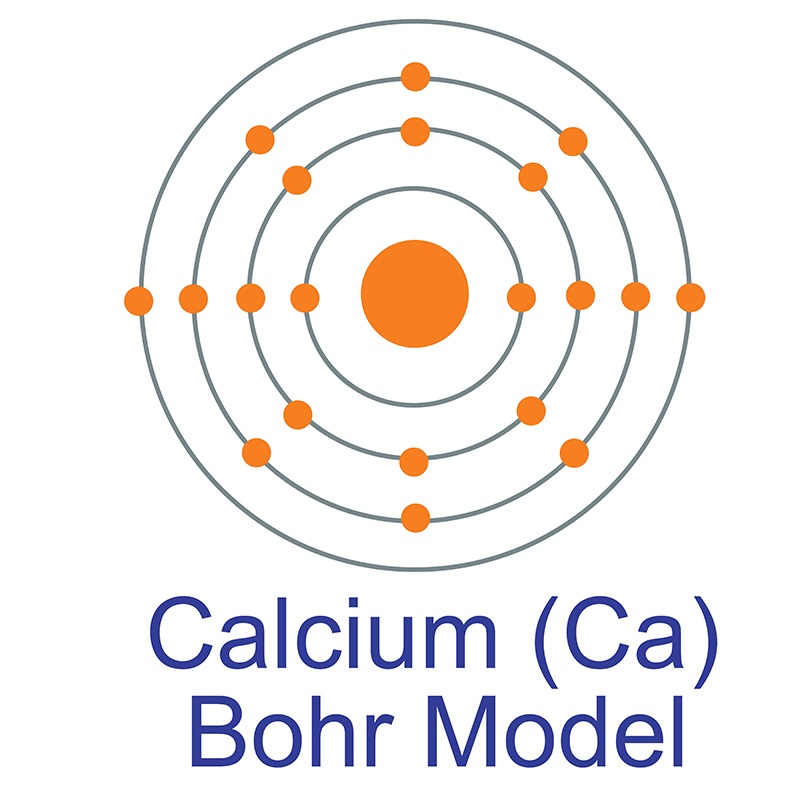
The Bohr Model of Calcium (Ca) has a nucleus that contains 20 neutrons and 20 protons. This nucleus is surrounded by four-electron shells named K-shell, L-shell, M-shell, and N-shell. The outermost shell in the Bohr diagram of Calcium contains only 2 electrons that also called valence electrons. Page Contents show
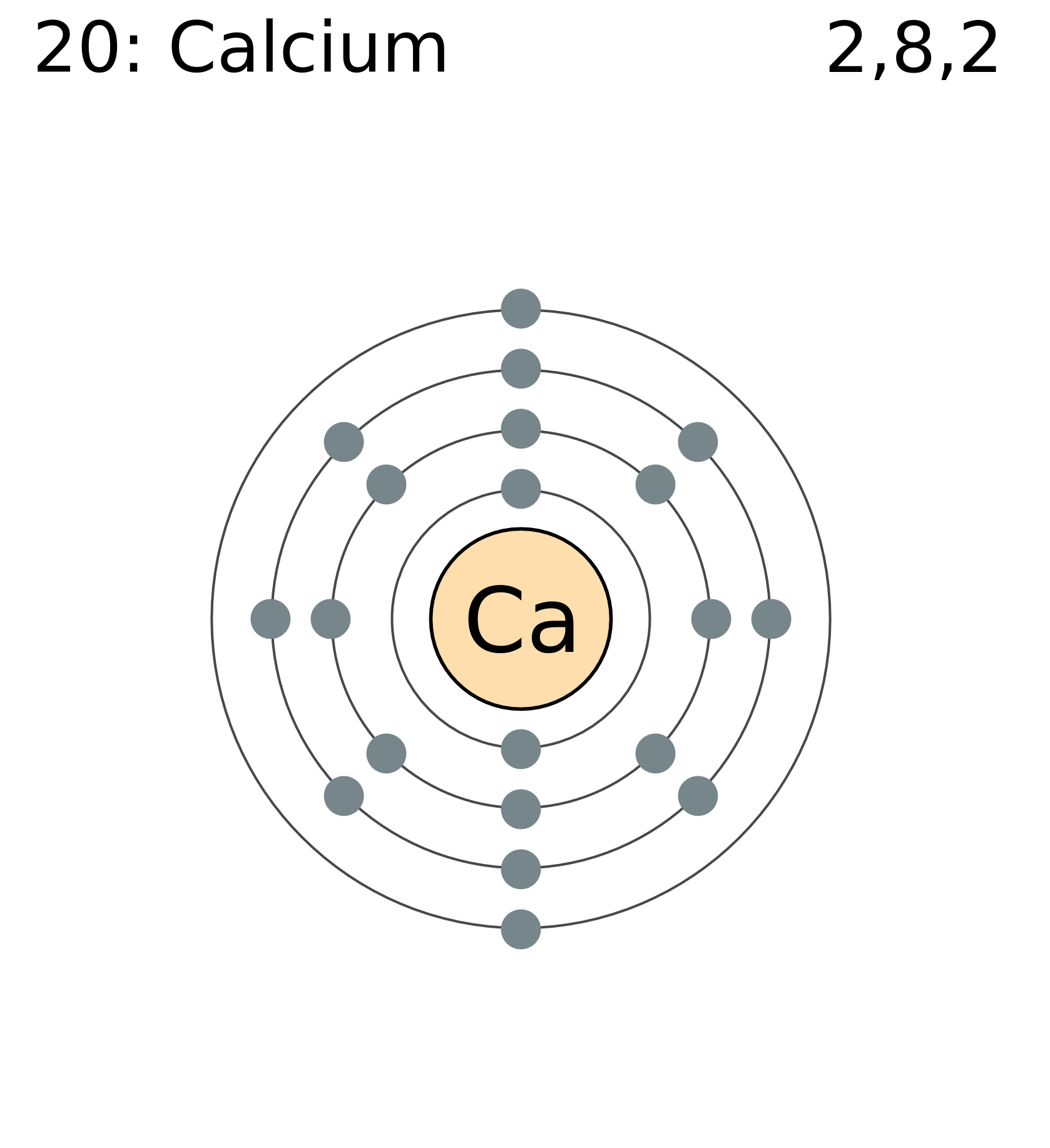
FileElectron shell 020 calcium.png
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.

Calcium Stock Photo Download Image Now iStock
Basic Information Name: Calcium Symbol: Ca Atomic Number: 20 Atomic Mass: 40.078 amu Melting Point: 839.0 °C (1112.15 K, 1542.2 °F) Boiling Point: 1484.0 °C (1757.15 K, 2703.2 °F) Number of Protons/Electrons: 20 Number of Neutrons: 20 Classification: Alkaline Earth Crystal Structure: Cubic Density @ 293 K: 1.55 g/cm 3 Color: Silvery
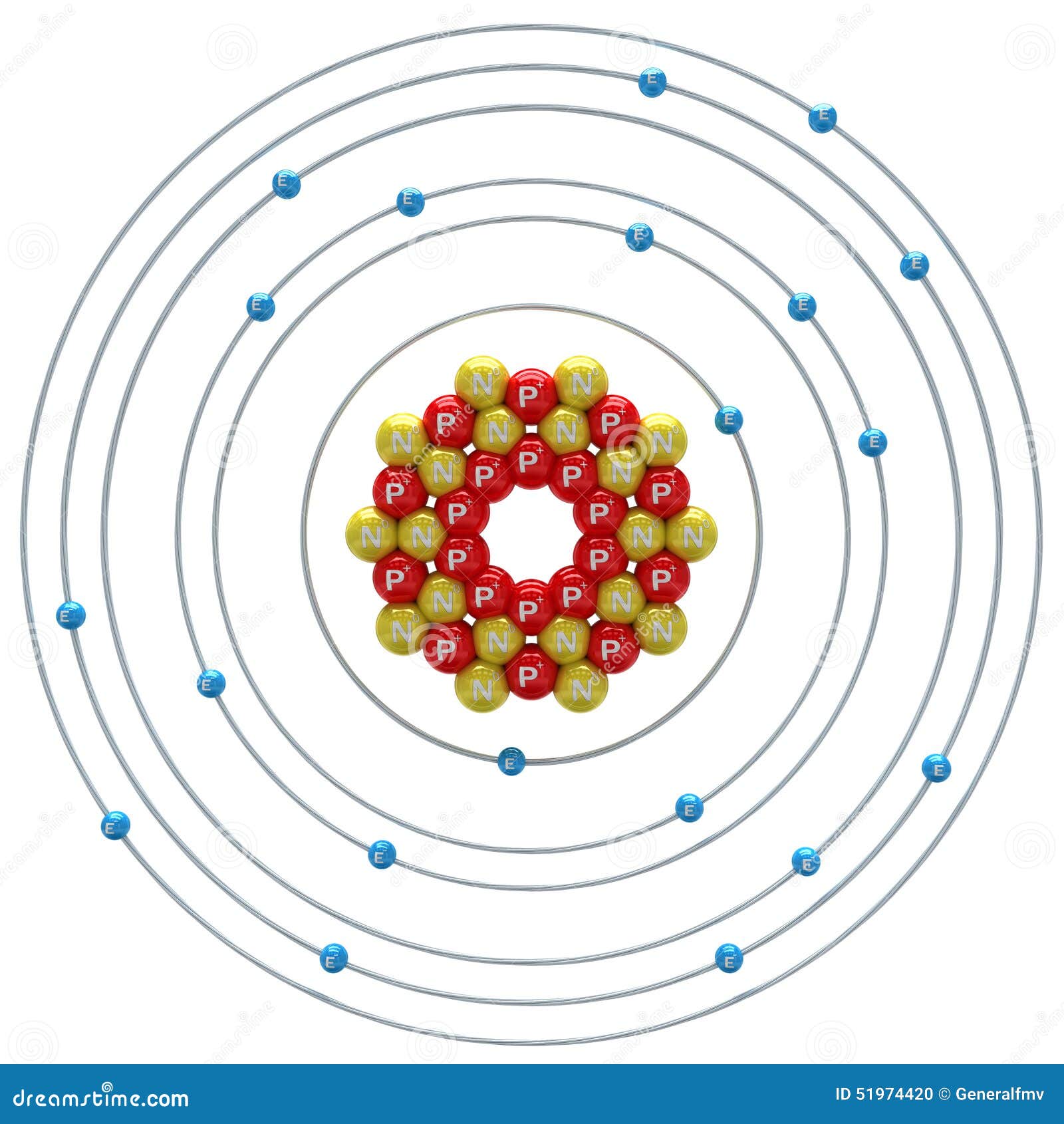
Calcium Atom on a White Background Stock Illustration Illustration of neutron, science 51974420
Calcium has 2 electrons in its first shell, 8 in its second, 8 in its third, and 2 in its fourth.Check me out: http://www.chemistnate.com

182 Calcium Atomic Properties Images, Stock Photos & Vectors Shutterstock
What is the Bohr model for calcium? A Visual Representation: A Bohr model is a way of visually representing what an atom of an element looks like. Atoms are tiny particles that make up.